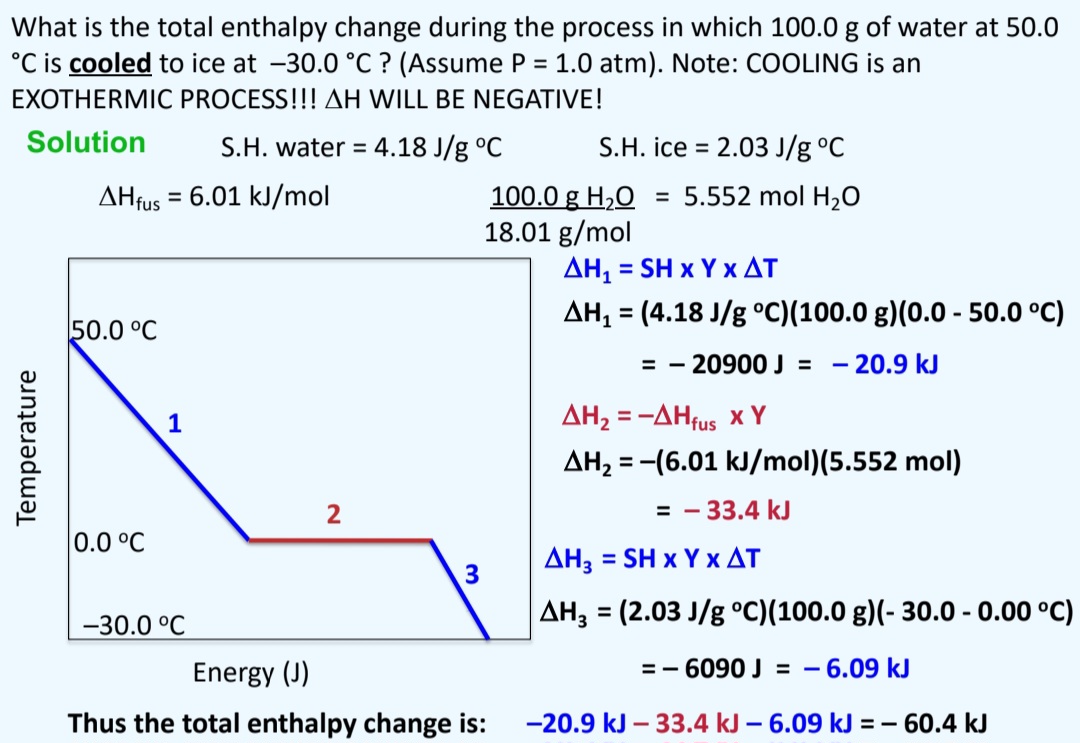
The Enthalpy Change For Converting 10.0 G Of Ice
The Enthalpy Change For Converting 10.0 G Of Ice
Hey there, future chemist! Are you wrestling with a chemistry problem about ice melting? Specifically, are you trying to figure out The Enthalpy Change For Converting 10.0 G Of Ice into liquid water? Don't worry, you're in the right place! This guide will break down the calculation step-by-step, making it super clear and easy to understand. By the end, you'll be a pro at solving these types of problems.
Understanding Enthalpy of Fusion
First off, let's get our terms straight. What exactly is 'enthalpy change'? In simple terms, it's the amount of heat absorbed or released during a chemical reaction or physical change. When ice melts, it absorbs energy from its surroundings.
The specific type of enthalpy change we're looking at here is the 'enthalpy of fusion'. This is the heat absorbed when one mole of a substance changes from a solid to a liquid phase at constant pressure. Think of it as the energy required to break those strong bonds holding the ice molecules in their rigid structure.
For our problem, understanding the enthalpy of fusion is crucial. It directly tells us how much energy is needed per mole to convert solid ice into liquid water. This value is a constant for water and forms the basis of our calculation for The Enthalpy Change For Converting 10.0 G Of Ice.
Key Data You'll Need
To calculate The Enthalpy Change For Converting 10.0 G Of Ice, we need a couple of fundamental pieces of information. These are standard values you'll often find in chemistry textbooks or data tables. Knowing where to find them and how to use them is half the battle!
The first piece of data is the molar mass of water (H2O). This tells us how many grams are in one mole of water. The second is the molar enthalpy of fusion for water, which we just discussed. Let's list them out clearly.
- Molar mass of H2O (water): Approximately 18.015 g/mol
- Molar enthalpy of fusion (ΔH_fus) for H2O: Approximately 6.01 kJ/mol
These two values, along with the given mass of ice, are all we need to perform our calculation. Make sure you always have these constants handy!
Step-by-Step Calculation for 10.0 g of Ice
Alright, now for the exciting part – the actual calculation! We're going to determine The Enthalpy Change For Converting 10.0 G Of Ice using the data we've gathered. Follow these steps carefully, and you'll get the correct answer every time.
Our strategy involves two main steps: first, converting the given mass of ice into moles, and then using the molar enthalpy of fusion to find the total enthalpy change. It's like building with LEGOs, one brick at a time!
- Calculate the moles of ice:
Moles = Mass / Molar Mass
Moles = 10.0 g / 18.015 g/mol ≈ 0.555 mol
- Calculate the total enthalpy change:
Enthalpy Change (ΔH) = Moles × Molar Enthalpy of Fusion
ΔH = 0.555 mol × 6.01 kJ/mol ≈ 3.336 kJ
So, The Enthalpy Change For Converting 10.0 G Of Ice is approximately 3.34 kJ (rounded to three significant figures). Remember, since it's an enthalpy of fusion (melting), the value is positive, indicating energy is absorbed.
And there you have it! You've successfully calculated The Enthalpy Change For Converting 10.0 G Of Ice into liquid water. We started by defining enthalpy of fusion, gathered our essential constants like molar mass and ΔH_fus, and then executed a simple two-step calculation. This process is fundamental in chemistry and helps us understand energy transformations in everyday phenomena. Keep practicing, and these problems will become second nature!
Frequently Asked Questions (FAQ)
- Q: What is enthalpy?
- A: Enthalpy is a measure of the total heat content of a system. The "enthalpy change" (ΔH) specifically refers to the amount of heat absorbed or released during a process at constant pressure.
- Q: Why is the enthalpy of fusion important in this calculation?
- A: The enthalpy of fusion is crucial because it quantifies the energy required to change a substance from its solid phase to its liquid phase. For our problem, it tells us how much energy is needed per mole to melt ice into water.
- Q: What if the mass of ice changes?
- A: If the mass of ice changes, you would simply substitute the new mass into the first step of the calculation (Moles = Mass / Molar Mass). The molar mass and molar enthalpy of fusion for water remain constant, so the rest of the steps would follow, yielding a different total enthalpy change.
- Q: Is the enthalpy change for melting always positive?
- A: Yes, for melting (fusion), the enthalpy change is always positive because energy must be absorbed from the surroundings to break the intermolecular forces holding the solid structure together. This is an endothermic process.
The Enthalpy Change For Converting 10.0 G Of Ice
The Enthalpy Change For Converting 10.0 G Of Ice Wallpapers
Collection of the enthalpy change for converting 10.0 g of ice wallpapers for your desktop and mobile devices.

Artistic The Enthalpy Change For Converting 10.0 G Of Ice Picture in 4K
Immerse yourself in the stunning details of this beautiful the enthalpy change for converting 10.0 g of ice wallpaper, designed for a captivating visual experience.

Amazing The Enthalpy Change For Converting 10.0 G Of Ice Moment in 4K
Explore this high-quality the enthalpy change for converting 10.0 g of ice image, perfect for enhancing your desktop or mobile wallpaper.

Detailed The Enthalpy Change For Converting 10.0 G Of Ice Abstract in 4K
Experience the crisp clarity of this stunning the enthalpy change for converting 10.0 g of ice image, available in high resolution for all your screens.
Dynamic The Enthalpy Change For Converting 10.0 G Of Ice Background Illustration
Find inspiration with this unique the enthalpy change for converting 10.0 g of ice illustration, crafted to provide a fresh look for your background.

Exquisite The Enthalpy Change For Converting 10.0 G Of Ice Background for Mobile
A captivating the enthalpy change for converting 10.0 g of ice scene that brings tranquility and beauty to any device.

Detailed The Enthalpy Change For Converting 10.0 G Of Ice Wallpaper in 4K
Transform your screen with this vivid the enthalpy change for converting 10.0 g of ice artwork, a true masterpiece of digital design.

Beautiful The Enthalpy Change For Converting 10.0 G Of Ice Background for Desktop
Experience the crisp clarity of this stunning the enthalpy change for converting 10.0 g of ice image, available in high resolution for all your screens.
Spectacular The Enthalpy Change For Converting 10.0 G Of Ice Design in 4K
Immerse yourself in the stunning details of this beautiful the enthalpy change for converting 10.0 g of ice wallpaper, designed for a captivating visual experience.

Lush The Enthalpy Change For Converting 10.0 G Of Ice View in 4K
Immerse yourself in the stunning details of this beautiful the enthalpy change for converting 10.0 g of ice wallpaper, designed for a captivating visual experience.

Gorgeous The Enthalpy Change For Converting 10.0 G Of Ice Photo Photography
A captivating the enthalpy change for converting 10.0 g of ice scene that brings tranquility and beauty to any device.
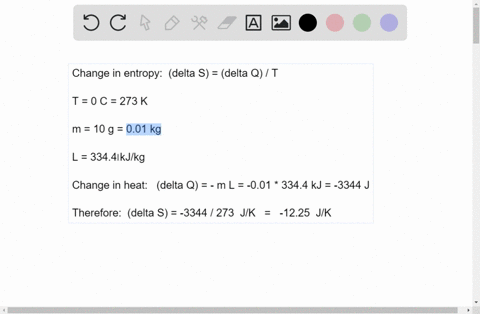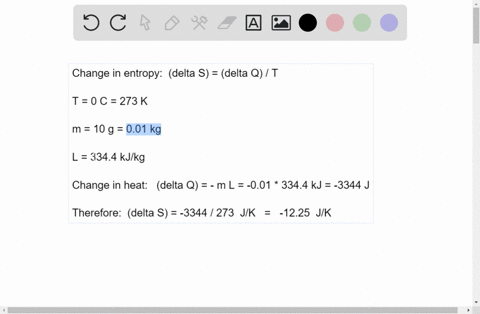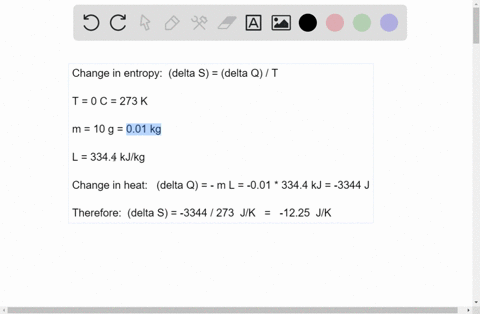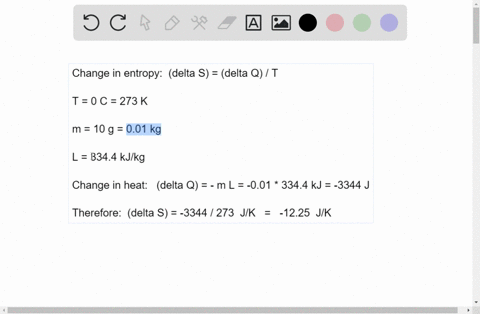
Mesmerizing The Enthalpy Change For Converting 10.0 G Of Ice Background for Mobile
Explore this high-quality the enthalpy change for converting 10.0 g of ice image, perfect for enhancing your desktop or mobile wallpaper.

Gorgeous The Enthalpy Change For Converting 10.0 G Of Ice Abstract for Your Screen
This gorgeous the enthalpy change for converting 10.0 g of ice photo offers a breathtaking view, making it a perfect choice for your next wallpaper.
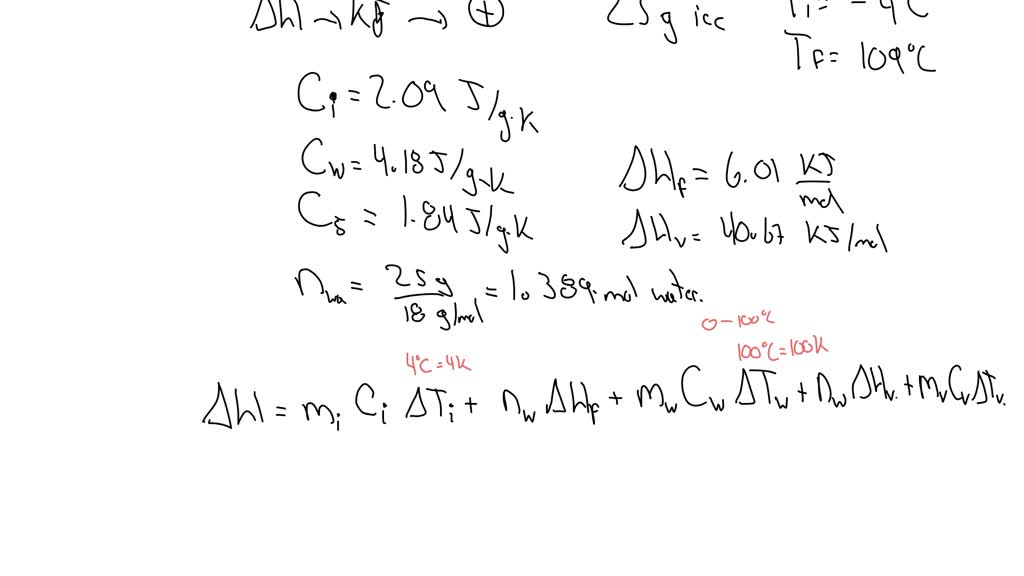
High-Quality The Enthalpy Change For Converting 10.0 G Of Ice Artwork Concept
Transform your screen with this vivid the enthalpy change for converting 10.0 g of ice artwork, a true masterpiece of digital design.

Dynamic The Enthalpy Change For Converting 10.0 G Of Ice View Illustration
Immerse yourself in the stunning details of this beautiful the enthalpy change for converting 10.0 g of ice wallpaper, designed for a captivating visual experience.

Artistic The Enthalpy Change For Converting 10.0 G Of Ice Photo for Your Screen
Find inspiration with this unique the enthalpy change for converting 10.0 g of ice illustration, crafted to provide a fresh look for your background.
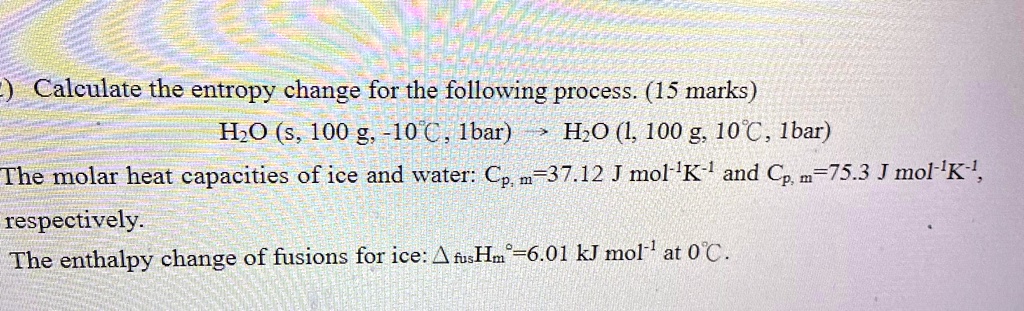
Crisp The Enthalpy Change For Converting 10.0 G Of Ice Image in HD
A captivating the enthalpy change for converting 10.0 g of ice scene that brings tranquility and beauty to any device.

Dynamic The Enthalpy Change For Converting 10.0 G Of Ice Image for Mobile
Immerse yourself in the stunning details of this beautiful the enthalpy change for converting 10.0 g of ice wallpaper, designed for a captivating visual experience.

Mesmerizing The Enthalpy Change For Converting 10.0 G Of Ice Capture Illustration
Transform your screen with this vivid the enthalpy change for converting 10.0 g of ice artwork, a true masterpiece of digital design.
Mesmerizing The Enthalpy Change For Converting 10.0 G Of Ice Moment Concept
Experience the crisp clarity of this stunning the enthalpy change for converting 10.0 g of ice image, available in high resolution for all your screens.

Beautiful The Enthalpy Change For Converting 10.0 G Of Ice Scene for Desktop
Explore this high-quality the enthalpy change for converting 10.0 g of ice image, perfect for enhancing your desktop or mobile wallpaper.
Download these the enthalpy change for converting 10.0 g of ice wallpapers for free and use them on your desktop or mobile devices.
0 Response to "The Enthalpy Change For Converting 10.0 G Of Ice"
Post a Comment